Use the Benefits of
CHO Production Media
MAM-PF®
Mammalian Artificial Cell Culture Media – Protein and Animal Component Free (ACF) for CHO, BHK, and other mammalian cells in accordance with the strict quality guidelines EMA/410/01.
Explore Growth and Density
MAM-PF® series
MAM-PF® is a production media. It is protein-free and protein hydrolysates free, chemically defined and for high cell density cultivation of a variety of cell lines such as CHO (Chinese Hamster Ovary) cells or BHK (Baby Hamster Kidney) cells and the high level expression of recombinant proteins. BioConcept holds a certificate for every single component used in the MAM-PF® media series to guarantee an untainted and exceptional final product.
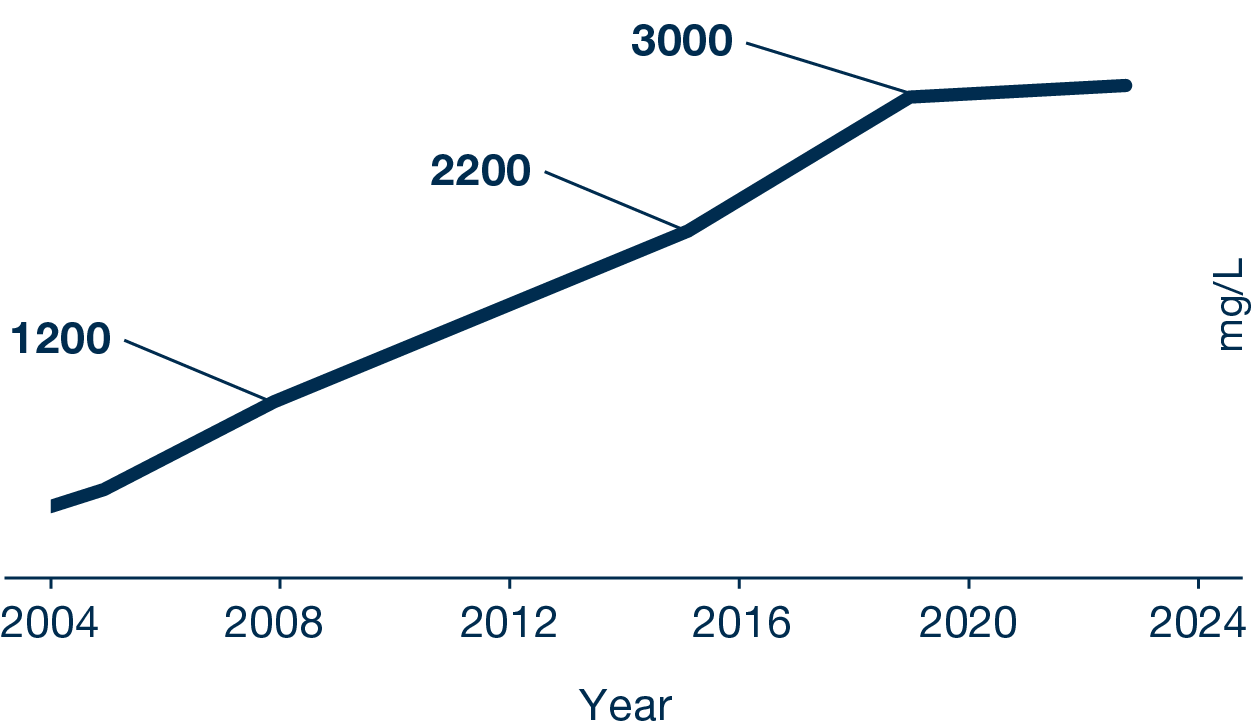
Development of MAM-PF®
Increase of Erythropoietin (EPO) yields during the system development. Within the last 4 years the product yield could be quadrupled up to 2.3 g/L using the MAM-PF77® medium and FMS3 in a fed-batch.
Animal Component Free
MAM-PF® media do not contain proteins or undefined hydrolysates.
Chemically defined
BioConcept holds TSE certificates for each component to ensure EMA/410/01 conformity.
Easy adaptation
In many cases it is possible to switch directly from your current medium to MAM-PF®.
High cell density | high product yield
Antibody production of up to 5.5 g/L
EPO of up to 2.5 g/L (see graph)
Cell density up to 3.7 x 107 cells/ml
Variable batch capacity
Liquid: 5 up to 5000 liter/day
Powder: 2 up to 800 kg/production run
Feed mixes
Various feed mixes are available for high density cell culture and high productivity.
Glycosylation
Best glycosylation pattern observe
Fast Growth
High Density
Cell density
CHOSI cells cultured in our media have shown fast growth and a much higher cell density compared to other media. This results in final product titers at the end of the fed-batch cycle. The higher viability in the stationary phase shows that the glycosylation is superior.
MAM-PF® and other Suppliers
Performance of MAM-PF77® and two different CHO media suppliers in a 14-day fed-batch mAb production.
FSH
A cell density of over 100 mio. cells per liter can be reached through mixing the CHO feed mix FMS3 to MAM-PF77®. It is possible to achieve a titer of over 350 mg/L of the highly glycosylated folliclestimulating hormone (FSH) within 14 days in a stirring bioreactor tank, making it a very high quality product. As determined during the purification process, 45% of the product showed an isoform-pattern, low aggregates, and low oxidized forms. MAM-PF77® can be used to produce quality FSH that fulfills its Ph. Eur. requirements.
FSH produced with MAM-PF® and FMS3
14-day bioreactor production scheme of the high glycosylated follicle-stimulating hormone (FSH) using MAM-PF77® and the FMS3 feed mix.
Ipilimumab — Antibody
The continuous innovation and development of the MAM-PF® media series has lead to the brand new MAM-PF77® cell culture medium and CHO Feed Mixes FMS3 and FMU. MAM-PF® media now increase productivity of Ipilimumab (monoclonal antibody) by up to 5g/L. The new expression system is also viable in fed-batch and perfusion systems.
Ipilimumab produced with MAM-PF® and FSU
High yields of mAbs, e.g. > 5g/L Iplimumab can be reached in a 14-day fed-batch system using MAM- PF77® plus the novel CHO feed mix FMU.
Biosimilars
Selection of pre-developed biosimilars produced with MAM-PF® media series.
All cell lines are propriety of EUGENEX Biotechnologies GmbH.
Molecule | Availability — Main Indication
Recombinant Proteins
EPO Epoetin alpha | Cell-Line | USP | DSP — Anemia
DPO Darbepoetin apha | Cell-Line | USP | DSP — Anemia
INFb Interferon beta-1a | Cell-Line | USP | DSP — Multiple Sclerosis
FSH Follicle Stimulating Hormone | Cell-Line | USP | DSP — Infertility
Human Choriogonadotropin (hCG) | Cell-Line | USP | DSP — Infertility
Human Luteinizing Hormone (hLH) | Cell-Line | USP | DSP — Infertility
Urokinase (uPA) | Cell-Line | USP — Thrombolysis
Alteplase (tPA) | Cell-Line | USP — Thrombolysis
Tenecteplase (TNK-tPA) | Cell-Line | USP | DSP — Thrombolysis
Factor VIIa (FVIIa) | Cell-Line | USP — Hemophilia
Factor VIII (FVIIII) | Cell-Line — Hemophilia
beta-Glucocerebrosidase (GCasebeta) | Cell-Line — Morbus Gaucher
Dornase alpha (DNAse I) | Cell-Line | USP — Cystic Fibrosis
Thrombin (FIIa) | Cell-Line — Hemostasis
Antibodies
Adalimumab (TNFa) | Cell-Line | USP | DSP — Arthritis, Psoriasis
Rituximab (CD20) | Cell-Line | USP | DSP — Lymphoma, Arthritis
Trastuzumab (HER2) | Cell-Line | USP | DSP — Breast & Gastric Cancer
Bevacizumab (VEGF) | Cell-Line | USP | DSP — Lung & Colorectal Cancer
Cetuximab (EGF-Receptor) | Cell-Line | USP | DSP — Colorectal & Head Cancer
Omalizumab (IgE) | Cell-Line | USP — Persistent Allergic Asthma
Denosumab (RANKL) | Cell-Line | USP | DSP — Osteoporosis
Tocilizumab (IL6-Receptor) | Cell-Line | USP | DSP — COVID-19, Arthritis
Ipilimumab (CTLA-4) | Cell-Line | USP | DSP — Lung & Renal Cancer
Panitumumab (EGF-Receptor) | Cell-Line | USP | DSP — Colorectal Cancer
Pertuzumab (HER2) | Cell-Line | USP — Breast Cancer
Eculizumab (Complement component 5) | Cell-Line | USP — Hemoglobinuria
Natalizumab (Integrin a) | Cell-Line | USP — Multiple Sclerosis
Infliximab (TNFa) | Cell-Line — Arthritis, Psoriasis
Atezolizumab (PD-L1) | Cell-Line — Urothelial & Breast Cancer
Daratumumab (CD38) | Cell-Line — Multiple Myeloma
Guselkumab (IL-23) | Cell-Line — Psoriasis
Fusion Proteins
Etanercept (TNFa/beta) | Cell-Line | USP | DSP — Chronical Arthritis
Abatacept (CD80, CD86) | Cell-Line | USP — Rheumatoid Arthritis
Belatacept (CD80, CD86) | Cell-Line — Kidney Transplantation
Dulaglutid (GLP-1-Receptor) | Cell-Line | USP | DSP — Diabetes mellitus Type II
Pembrolizumab (PD1-Receptor) | Cell-Line | USP — Lung & Renal Cancer
Nivolumab (PD1-Receptor) | Cell-Line | USP — Melanoma, Lung Cancer
Downloads:
References:
Do you need more information?
Don’t hesitate to ask. We are glad to help!